There are some topics in the wine world that are more confusing than others. One of these topics is “wine diamonds.” These are tiny crystals sprouting out of your wine cork or settling at the bottom of your wine bottle. They may look like small chards of glass, but they are something very different and not at all dangerous to consume. Wine diamonds are actually bits of tartaric acid that have settled out of the wine.

Grapes contain three main acids; malic, citric and tartaric. The levels of these acids change as the berry goes through maturation. Each one of these acids are responsible for providing different flavor profiles and mouthfeel to the wine.
Malic acid levels are more predominant in cooler climates and decreases during maturation as the berry continues to undergo respiration. This acid is most commonly discussed during malolactic fermentation. During secondary, or malolactic fermentation, lactic acid bacteria convert essentially 100% of remaining natural malic acid by reducing it to lactic acid. Winemakers choose to allow the wine to go through malolactic fermentation in order to produce a softer, smoother wine.
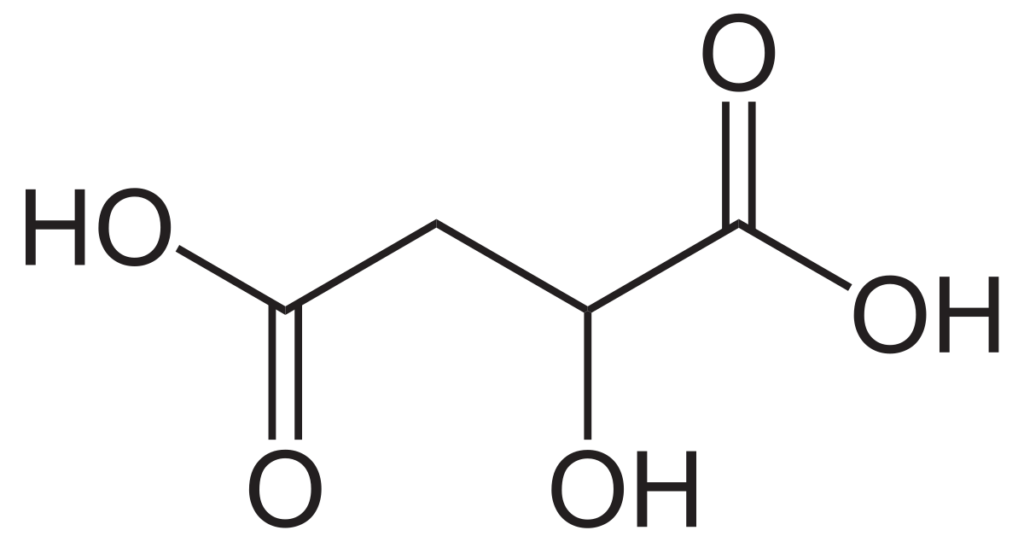
Found significantly less in grapes is citric acid. This is produced by the yeast during fermentation, with certain yeast species releasing more than others. Citric acid is also broken down during malolactic fermentation. The process produces diaceytl, which is that butter flavor some Chardonnays are known for. However, it also can be converted to acetic acid by unwanted lactic acid bacteria, increasing the level of volatile acidity in the wine.
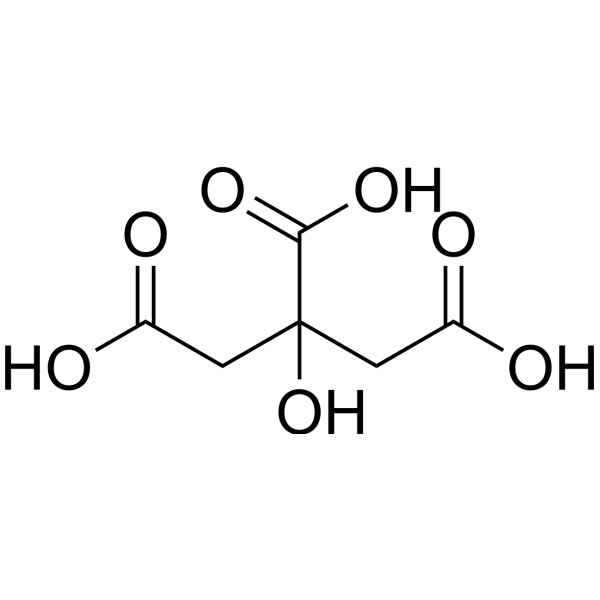
Tartaric acid levels vary by variety. Unlike malic acid, tartaric acid levels do not decline during ripening. Since it is difficult to metabolize by a majority of microorganisms, it increases the acidity of high pH wines. But for the same reason, its presence runs the risk of increasing bitartrate instability.
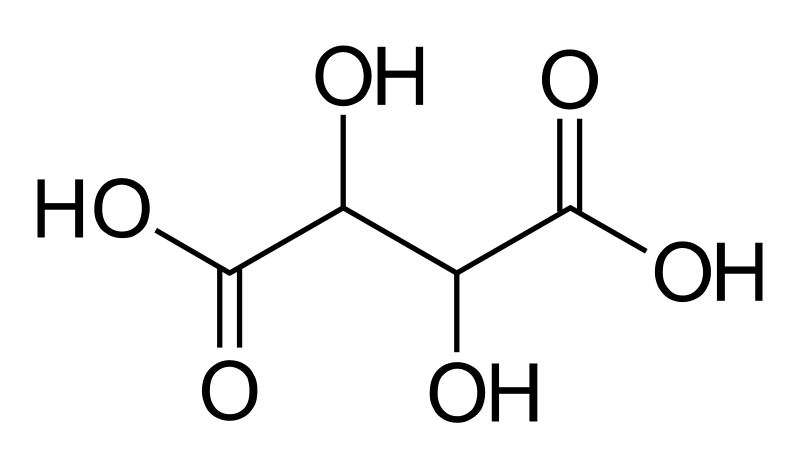
While fermentation and aging occurs, some of the tartaric acid will naturally settle out binding itself to the lees, pulp, tannins and pigments. However much will remain soluble, which is great as it increases the mouthfeel and balance of the wine and increases complexity. As wine ages, the dissolved tartrates begin to crystalize and as they increase in size, they begin to fall out of suspension.
The solubility of tartaric acid is temperature dependent. The teetering point is typically around 40 degrees Fahrenheit. At this temperature, the acid binds with potassium that naturally occurs in wine to form the crystal deposits; potassium bitartrates (tartrates for short.)
We typically see the crystals, aka wine diamonds, more in white wines as the temperature plays a role in the speed of the crystallization. Cooler temperatures speed up the process and white wines are more often served chilled. Winemakers can eliminate the potential of wine diamonds by choosing to put their wines through a process known as cold stabilization. Prior to bottling, the wine’s temperature is dropped to near freezing for several days while in a stainless steel tank. This forces the potassium bitartrates to crystalize and settle to the bottom. The wine is then filtered in order to capture the crystals.
Have you been missing the weekly Exploring the Wine Glass posts? They have moved. Sign up at http://eepurl.com/be49CD to never miss a post. Subscribe to this blog in the sidebar on this page.
As there is no harm to the wine or to the consumer to ingest the crystals, this process is done solely for aesthetics. The positive is that the wine is crystal clear, however cold stabilization is known to strip the wine of acidity as well as extracting some of the natural flavors. Since the acidity is lowered, it also impacts the ageability of the wine.
What do you do if you find wine diamonds in your wine? You don’t need to do anything. But if you feel obligated to lower the likelihood of finding them, keep in mind that refrigerators are really too cold. The average refrigerator temperature is 37 degrees Fahrenheit, increasing the likelihood of crystals forming. Instead store the wine on its side between 55 and 60 degrees Fahrenheit and pop the wine in the refrigerator about a half hour prior to opening to bring its temperature down between 45 and 48 degrees Fahrenheit.
If you do find some wine diamonds in your bottle, and don’t want them in your glass, you can either allow the crystals to settle to the bottom and decant the wine slowly while pouring at a slight angle. You can alternately filter the wine through a cheesecloth or a coffee filter to capture any diamonds.
~Slàinte!
Please follow us on Instagram, Twitter, Facebook and Youtube.
We’ve stacked the odds so that you can get our award winning wines without breaking the bank. Click the image to find out all of the benefits of joining the CHALK CLUB including discounted shipping and up to 25% off all purchases. .




